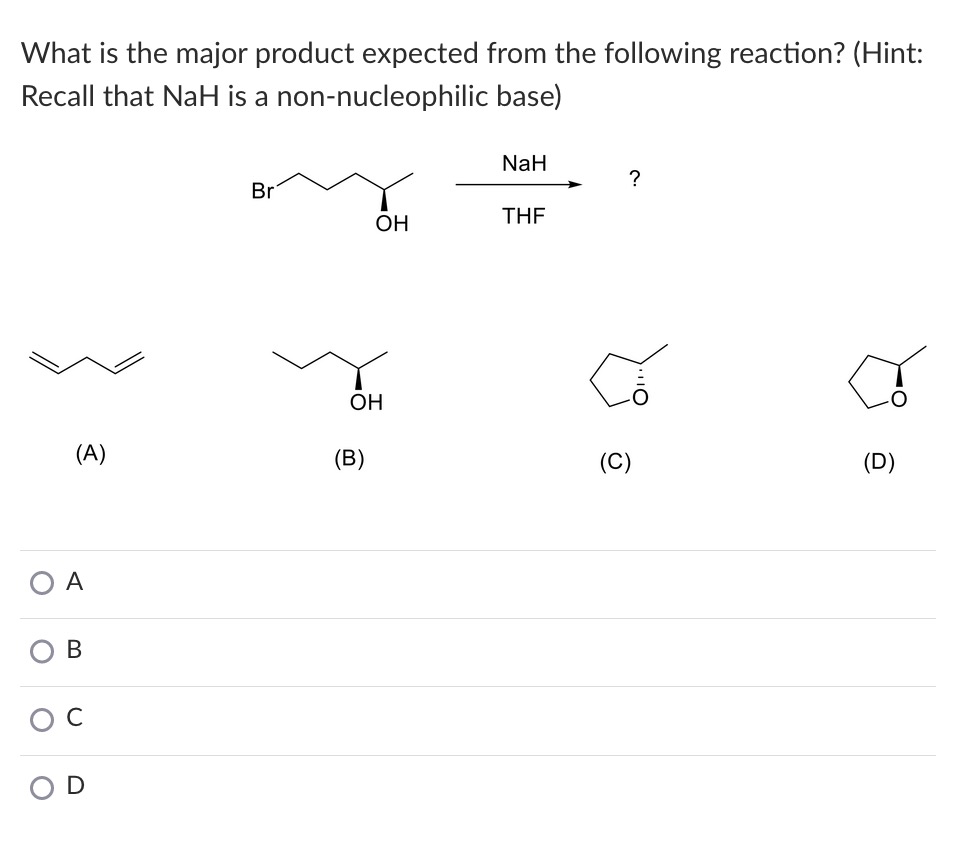
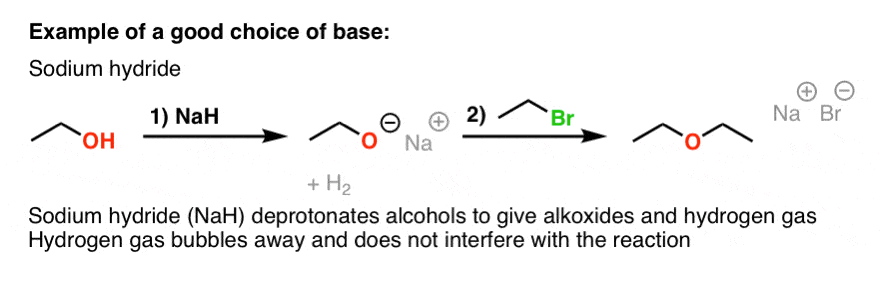
I'm having trouble determining what the base in this E1 reaction is , anyone has an idea ? : r/OrganicChemistry

OneClass: Predict the structures of BOTH bronsted acid base reaction of NaH with typical thiol. What ...

The hydride ion, H^- is a stronger base than its hydroxide ion OH^- . Which of the following reactions will occur, if sodium hydride (NaH) is dissolved in water?

The hydride ion H^(-) is a stronger base than its hydroxide ion OH^(-). Which of the following reactions will occurs if sodium hydride (NaH) is dissolved in water ?
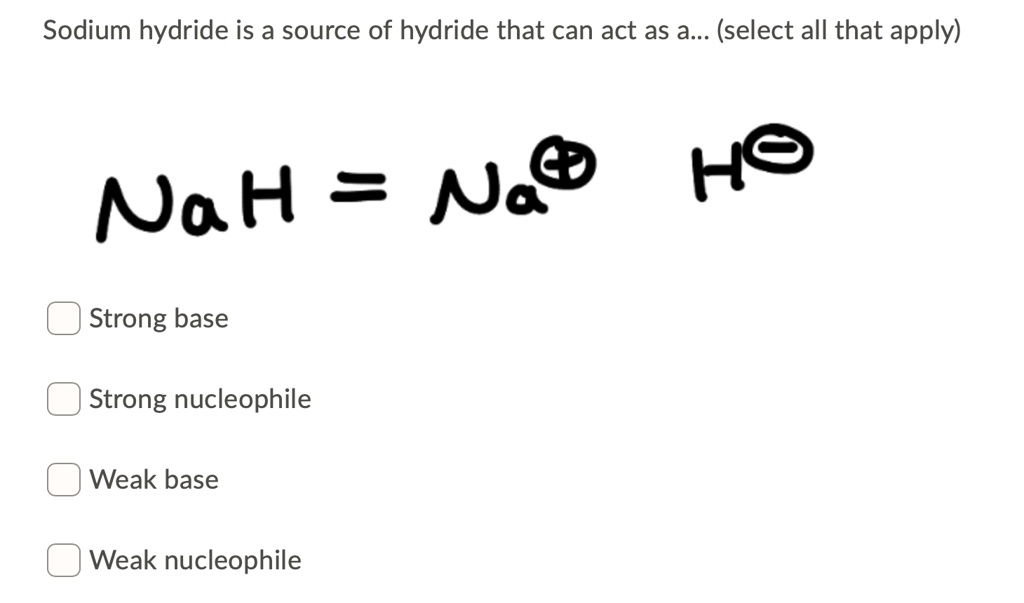
SOLVED: Sodium hydride is a source of hydride that can act as a (select all that apply) Na@ HO NaH = Strong base Strong nucleophile Weak base Weak nucleophile
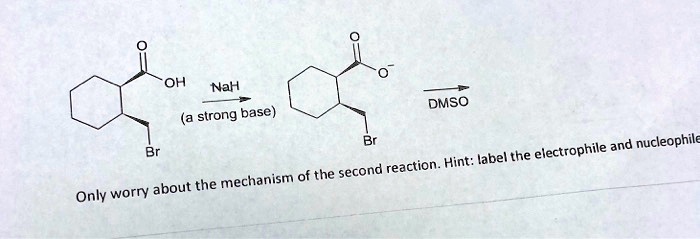
SOLVED: OH NaH DMSO strong base) and nucleophile Hint: label the electrophile second reaction mechanism of the Only worry about the

Understanding the Origins of Nucleophilic Hydride Reactivity of a Sodium Hydride–Iodide Composite - Hong - 2016 - Chemistry – A European Journal - Wiley Online Library