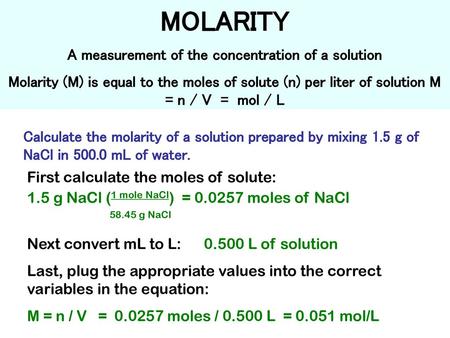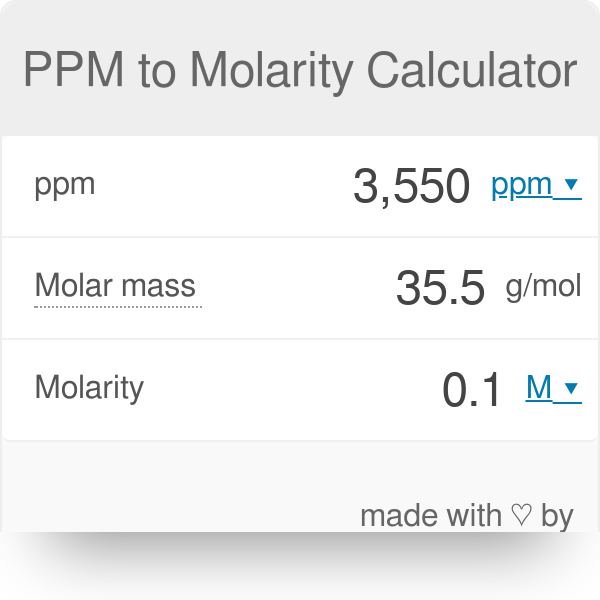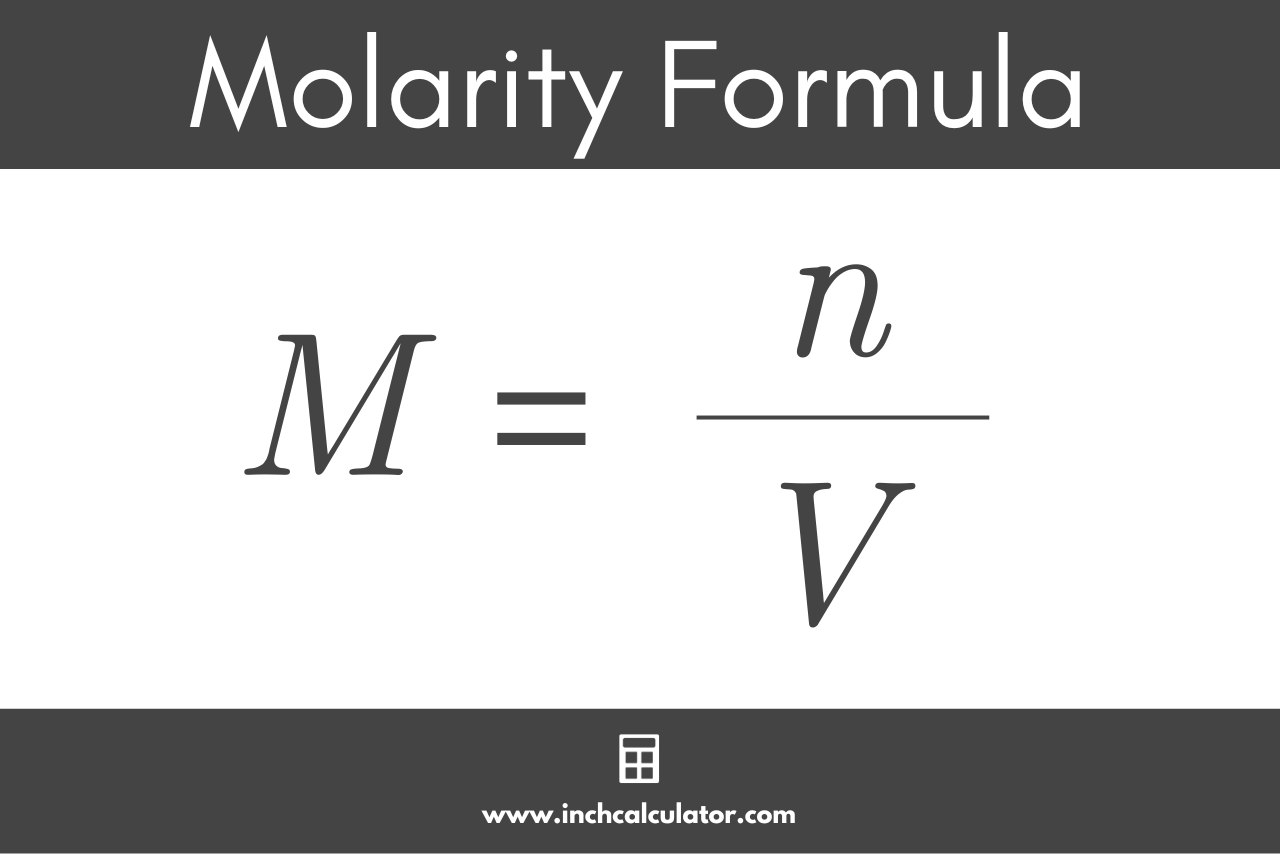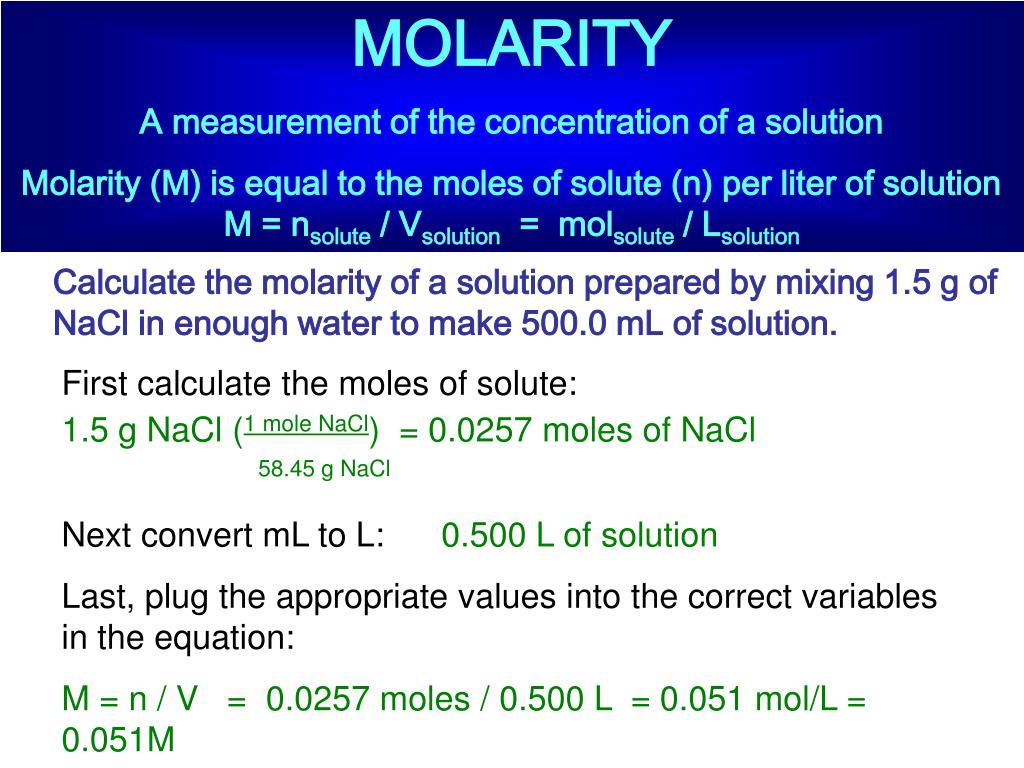
PPT - MOLARITY A measurement of the concentration of a solution PowerPoint Presentation - ID:1459950
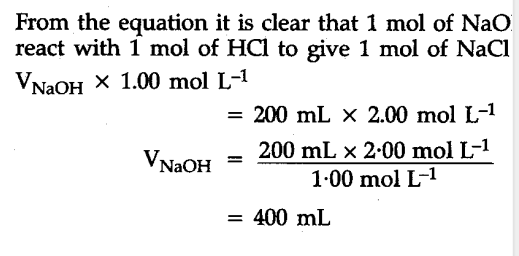
Calculate the volume of 1.00 mol ${{L}^{-1}}$ aqueous sodium hydroxide that is neutralised by 200 mL of 2.00 mol ${{L}^{-1}}$ aqueous hydrochloric acid arid the mass of sodium chloride produced - CBSE
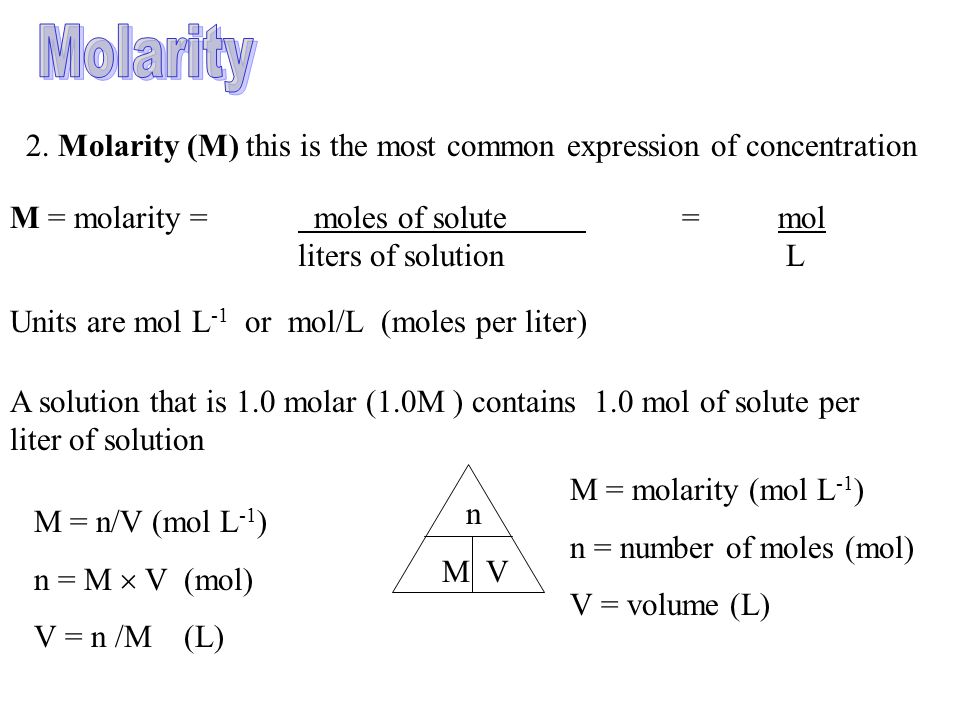
Molarity 2. Molarity (M) this is the most common expression of concentration M = molarity = moles of solute = mol liters of solution L Units are. - ppt download