
How to Balance H2SO4 + B(OH)3 = B2(SO4)3 + H2O | How to Balance H2SO4 + B(OH)3 = B2(SO4)3 + H2O | By Organic Chemistry Tutorial/Inorganic Chemistry/Science | Facebook

How to Balance Al2O3 + H2SO4 = Al2(SO4)3 + H2O,How to Balance Aluminum oxide + Sulfuric acid,How to Balance Aluminum oxide + Sulfuric acid + Aluminum sulfate + Water,balancing Al2O3 + H2SO4 =
How many unpaired electrons are present in the Brown Ring complex [Fe(H2O )5(NO)]SO4 (1) 4 (2) 3 (3) 0 (4) 5
![SOLVED: [Cu(NH3)4]SO4 • H20 (s) was prepared from 15.5 grams CuSO4 • 5 H2O (s) using a total of 10 mL of 15 M NH3. Determine which reactant is limiting. Molar Masses: SOLVED: [Cu(NH3)4]SO4 • H20 (s) was prepared from 15.5 grams CuSO4 • 5 H2O (s) using a total of 10 mL of 15 M NH3. Determine which reactant is limiting. Molar Masses:](https://cdn.numerade.com/ask_previews/3e5daa89-75dc-40aa-92e7-425d1e093c86_large.jpg)
SOLVED: [Cu(NH3)4]SO4 • H20 (s) was prepared from 15.5 grams CuSO4 • 5 H2O (s) using a total of 10 mL of 15 M NH3. Determine which reactant is limiting. Molar Masses:
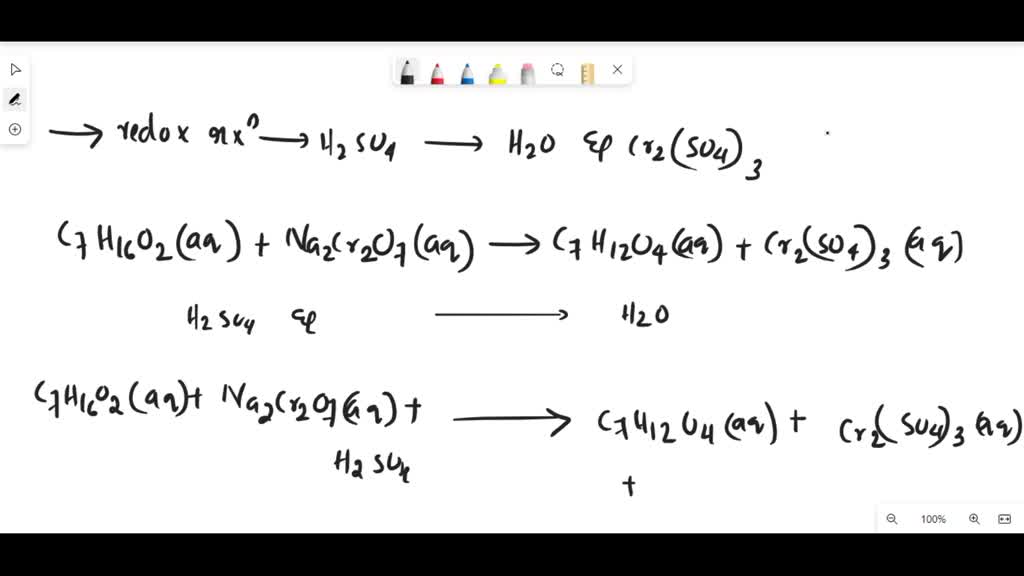
SOLVED: Balance the following redox reaction if it occurs in H2SO4. What are the coefficients in front of H2O and Cr2(SO4)3 in the balanced reaction? C6H14O2(aq) + K2Cr2O7(aq) → C6H10O4(aq) + Cr2(SO4)3(aq)









