![Cp∗Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes - ScienceDirect Cp∗Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1381117702001649-gr1.gif)
Cp∗Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes - ScienceDirect

Calcualte the enthalpy change on freezing of 1.0 mole of water at 10.0^(@)C to ice at -10^(@)C. ... - YouTube
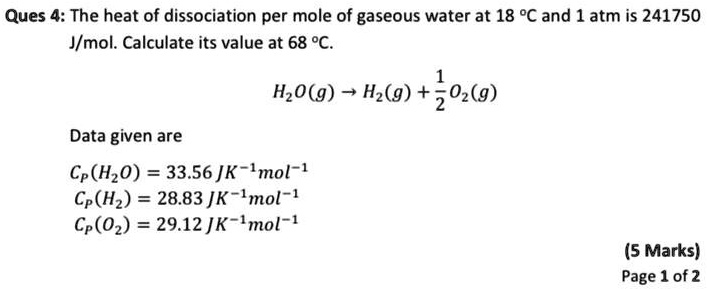
SOLVED: Ques 4: The heat of dissociation per mole of gaseous water at 18 %C and 1 atm is 241750 Jmol. Calculate its value at 68 %. HzO(g) Hz(g) + 202(9) Data

The enthalpy change for a reaction at equilibrium is - 20.5 kJ mol ^-1 . Then the entropy change for this equilibrium at 410 K is:
![Cp*Rh(bpy)(H2O)]2+ as a coenzyme substitute in enzymatic oxidations catalyzed by Baeyer–Villiger monooxygenases - Chemical Communications (RSC Publishing) Cp*Rh(bpy)(H2O)]2+ as a coenzyme substitute in enzymatic oxidations catalyzed by Baeyer–Villiger monooxygenases - Chemical Communications (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/B504921K)



![Solved Heat Capacities pIH20l 37.65 J/K-mol Cp[H2O(l)]-75.29 | Chegg.com Solved Heat Capacities pIH20l 37.65 J/K-mol Cp[H2O(l)]-75.29 | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F9c6%2F9c6322fb-d755-40a1-8987-9484af98164f%2FphpxcNGUz.png)