![Welcome to Chem Zipper.com......: How much ice will separate if a solution containing 25 g of ethylene glycol [C2H4(OH) 2] in 100g of water is cooled to 10°C? Kf(H2O) = 1.86 (25.05 Welcome to Chem Zipper.com......: How much ice will separate if a solution containing 25 g of ethylene glycol [C2H4(OH) 2] in 100g of water is cooled to 10°C? Kf(H2O) = 1.86 (25.05](https://lh3.googleusercontent.com/-l-d6OtTLXJA/XtYl5wlD-9I/AAAAAAAAHts/sC9TM2xK2iMGbrCcNXPwk7RHqGIui06GwCLcBGAsYHQ/w1200-h630-p-k-no-nu/1591092699610513-0.png)
Welcome to Chem Zipper.com......: How much ice will separate if a solution containing 25 g of ethylene glycol [C2H4(OH) 2] in 100g of water is cooled to 10°C? Kf(H2O) = 1.86 (25.05

SOLVED: hi i was wondering if anyone can explain how many grams of ethylene glycol C2H4(OH)2, are needed per kilogram of water to protect radiator fluid against freezing down to 0°F?
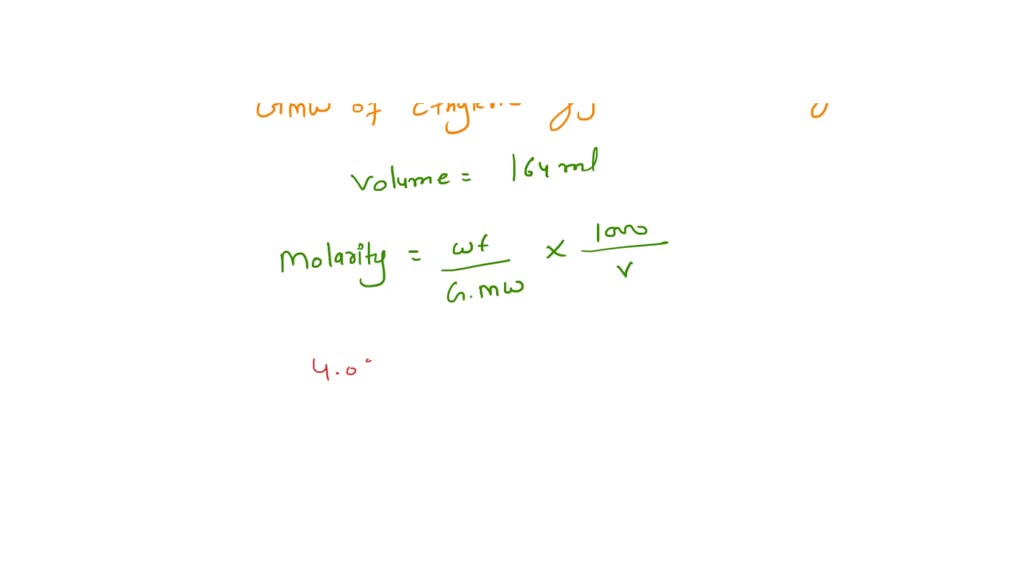
SOLVED: 1. Ethylene glycol is the primary component in antifreeze. How many grams of ethylene glycol (C2H4(OH)2) are present in 164 mL of a 4.024 M solution of antifreeze? 2. According to
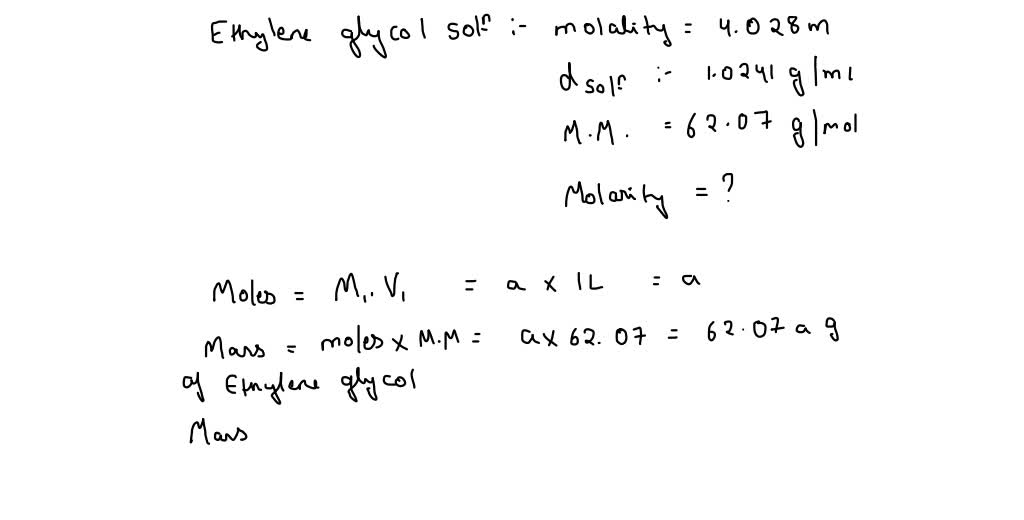





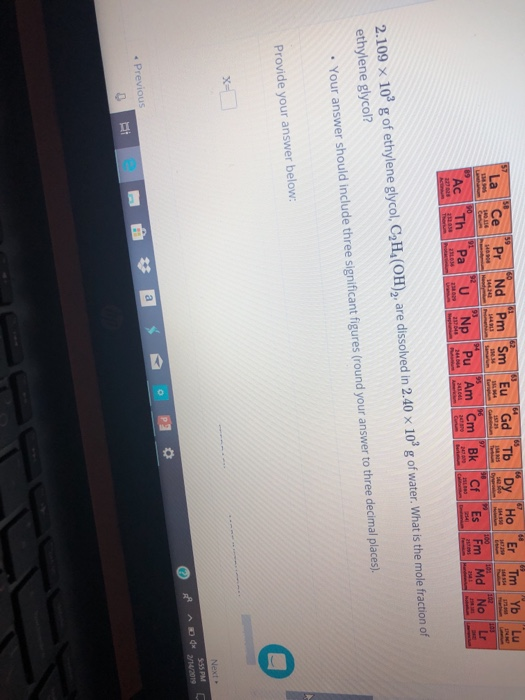





![Solved 1) 5.00 kg glycol, C2H4(OH)2, [this is antifreeze!] | Chegg.com Solved 1) 5.00 kg glycol, C2H4(OH)2, [this is antifreeze!] | Chegg.com](https://media.cheggcdn.com/study/d59/d59c3bd9-c862-4870-b923-e50820a495c3/image)